Glendale, CA,Oct 7, 2016
Nestlé USA, Inc. is initiating a voluntary recall of its Nestlé Drumstick Club 16 count Variety Pack and 24 count Vanilla Pack (with cones marked for easy individual sale) due to a possible health risk. The two pack sizes contain 4.6 fl.oz. cones and were manufactured in Bakersfield, Calif. and distributed nationally. No other production codes, sizes or varieties of Nestlé Drumstick products are affected by this recall.
The company received positive test results for Listeria monocytogenes (LM) from equipment contact surfaces from a location on the production line where these products are made. There have been no positive test results for LM present in the Drumstick cones themselves. The products impacted by the voluntary recall were put into distribution inadvertently. No illnesses have been reported to date; the company is initiating this recall as a precautionary action to avoid any potential for consumer illness.
Listeria monocytogenes can cause serious and sometimes fatal infections in young children, frail or elderly people, and others with weakened immune systems. Although healthy individuals may suffer only short-term symptoms such as high fever, severe headache, stiffness, nausea, abdominal pain and diarrhea, Listeria infection can cause miscarriages and stillbirths among pregnant women.
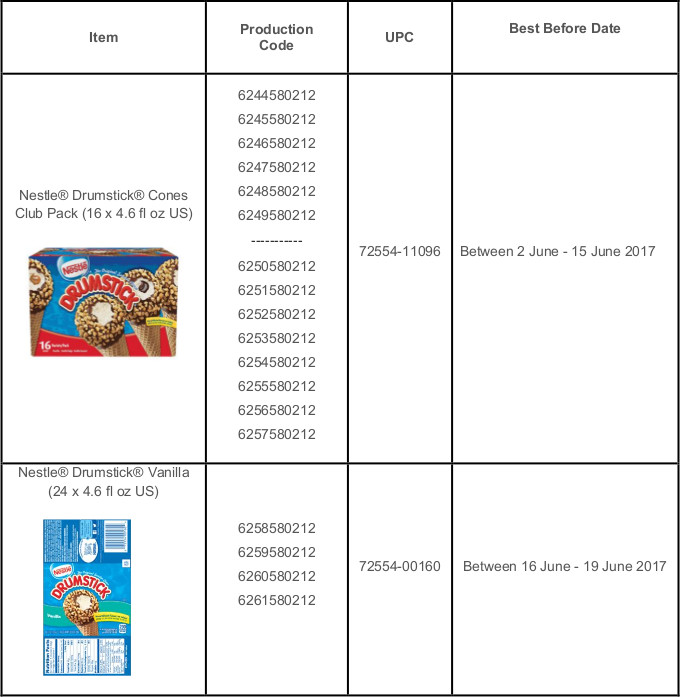
The Nestlé recall is limited to the Drumstick Club 16 Count Variety Pack and 24 count Vanilla Pack, made at the company’s Bakersfield, Calif. ice cream production facility. The product identification codes can be found on the back of the packages and on the individually marked vanilla cones from the 24 count pack. The two packs being recalled carry distinct UPC codes, as well as a “best before” date and production code.
Consumers who may have purchased the product(s) listed above should not consume it, but instead should return it to the place of purchase or contact Nestlé Consumer Services for replacement. Please call or text 1-800-681-1676 or email [email protected]; representatives are available 24/7. News about this recall can also be found on Drumstick.com.
The quality and safety of our products remain our number one priority. We apologize for any inconvenience this action represents for both our consumers and retail customers.
Q&A
Which products are being recalled and why?
Nestlé USA, Inc. is recalling two SKUs of its Nestle® Drumstick® ice cream products. Two products are being recalled due to a possible health risk:
- Nestlé Drumstick Club 16 ct. Variety Pack, which is a Club Pack sold in the Club Channel and contains Vanilla, Vanilla Fudge and Vanilla Caramel flavors.
- Nestlé Drumstick 24 ct. pack that contains Single Vanilla Cones that are meant for individual sale and sold at convenience stores such as 7-11.
We are recalling the products out of an abundance of caution because equipment contact surfaces tested positive for Listeria monocytogenes.
Have you found Listeria in the product itself?
No. There have been no positive test results for Listeria monocytogenes present in the Drumstick cone products themselves. However, the company has received positive test results for Listeria monocytogenes on equipment contact surfaces located on the production line where these items were made. Therefore, the company is taking precautionary action to recall the items to avoid any potential for consumer illness. No other production codes, sizes or varieties of Nestlé Drumstick products are affected by this recall.
What are the symptoms of Listeria?
Listeria monocytogenes is an organism which can cause serious and sometimes fatal infections in young children, frail or elderly people, and others with weakened immune systems. Although healthy individuals may suffer only short-term symptoms such as high fever, severe headache, stiffness, nausea, abdominal pain and diarrhea, Listeria infection can cause miscarriages and stillbirths among pregnant women.
How did this happen?
Nestlé conducts a “test and hold” program and identified this potential problem through its own testing protocol. Unfortunately, an error occurred in logging receipt of the test result and the product in question was inadvertently shipped to retailers.
What are you doing to prevent a similar occurrence?
We’re now looking into reinforcing our checks and balances so that such errors do not reoccur. We’re also investigating the root cause of the finding.
How often do you clean your equipment? You mentioned that listeria was found on an equipment contact surface?
We do a full clean after every day’s production.
Why did it take so long for you to find this?
Unfortunately, an error occurred in logging receipt of the test result. We discovered the error during a subsequent review of records. As soon as we identified the error, we notified FDA and initiated the recall.
This sounds like another Blue Bell situation with listeria in ice cream. Is your situation the same as theirs was?
No. Each recall has its own unique facts. Except for the coincidence that our recall involved both ice cream and listeria, our situation is much different from Blue Bell’s in a number of significant ways, including: (1) we have received no reports of human illnesses; (2) we have no listeria findings in the ice cream itself (just the equipment); (3) we have only one product line affected; (4) we have only one facility affected; and (5) we self-identified this event and took precautionary steps to recall product.
Will there now be a shortage of these products?
There may be a temporary shortage as our retail customers remove the recalled products from sale and we work to clear the distribution channel of affected products.
When were the products produced?
The products were produced at our Bakersfield, California facility between August 31 and September 17, 2016.
What should consumers do if they have questions about the recall?
Consumers who have these products should not consume them, and should return it to the place of purchase or contact Nestlé Consumer Services for replacement. Consumers can call/text 1-800-681-1676 or email [email protected].