HAMPTON, Va., April 18, 2017 /PRNewswire/ — There were 149,933 food facilities registered with the U.S. Food and Drug Administration (FDA) as of February 2, 2017. Of those registered facilities, 70,976 (47%) were outside of the United States. See the number of registrations by country here.
The ten countries with the largest number of FDA registered food facilities remain the same in 2017 as those reported in 2016:
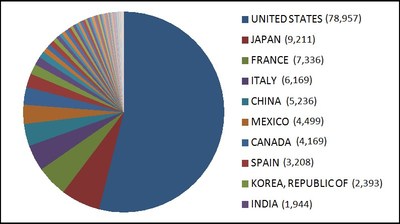
- United States (78957)
- Japan (9211)
- France (7336)
- Italy (6169)
- China (5236)
- Mexico (4499)
- Canada (4169)
- Spain (3208)
- Republic of Korea (2393)
- India (1944)
Earlier research revealed there were 207,653 food facilities registered with FDA on January 1, 2016, meaning there was a 28% decrease in the number of registered facilities between then and February 2017. This steep drop in registered facilities is likely due to FDA removing food facility registrations that were not properly renewed by December 31, 2016 from its registration database
This drop in registered facilities is greater than the 14% drop that occurred after the 2014 renewal period. This is likely due to new verification requirements implemented during the 2016 renewal period. Food facilities located outside of the United States are required to designate a U.S. Agent for FDA Communications in their registration renewal. Unlike in previous years, individuals or entities listed as U.S. Agents in 2016 were required to confirm with FDA acceptance of their designation and corresponding responsibility. FDA did not consider a facility’s 2016 renewal confirmed unless the designated U.S. Agent affirmatively agreed in writing. If a foreign facility submitted its registration renewal but its U.S. Agent did not affirmatively agree, its registration was removed from FDA’s food facility registration database.
Manufacturing, processing, packing, or storing food for US consumption without a valid FDA registration is a prohibited act. Many facilities do not realize that FDA removed their registrations until problems occur. To avoid costly detentions or regulatory action, it’s prudent for all food facilities to verify that their FDA registrations were properly renewed for 2017 before continuing with business as usual. This is especially true for food facilities located outside the U.S. Registrar Corp will verify that your FDA food facility registration was properly renewed at no cost. Facilities that need a U.S. Agent may retain Registrar Corp. Simply contact Registrar Corp at +1-757-224-0177 or chat with a Regulatory Advisor 24-hours a day at www.registrarcorp.com/livehelp.
Contact: David Lennarz, Vice President, Registrar Corp
+1-757-224-0177, [email protected]
SOURCE Registrar Corp







