By Tiffany Donica, Customer Success Coach at SafetyChain Software
The finalization of the Food Safety Modernization Act (FSMA) Rule 204(d) is rapidly approaching. In November 2022, a key piece of FSMA will come into play for many manufacturers. Known as the Food Traceability Proposed Rule, the goal of 204 is to create visibility within the supply chain to enable a better response to foodborne illnesses, contamination, and other public health and safety issues. Produce manufacturers preparing to work with traceability beyond their individual place within the chain are poised to take an active part in shaping future policy. In addition, proactively integrating traceability within a more extensive food safety plan can help insulate against risks.
Let’s review the fundamentals and goals of FSMA 204 and how you can adapt.
The Fundamentals of FSMA 204
The Food Traceability Proposed Rule establishes the Food Traceability List (FTL), a list of foods for which the FDA requires additional recordkeeping. It also defines the requirements for how the recordkeeping will take place and who and what is exempt from this updated traceability method.
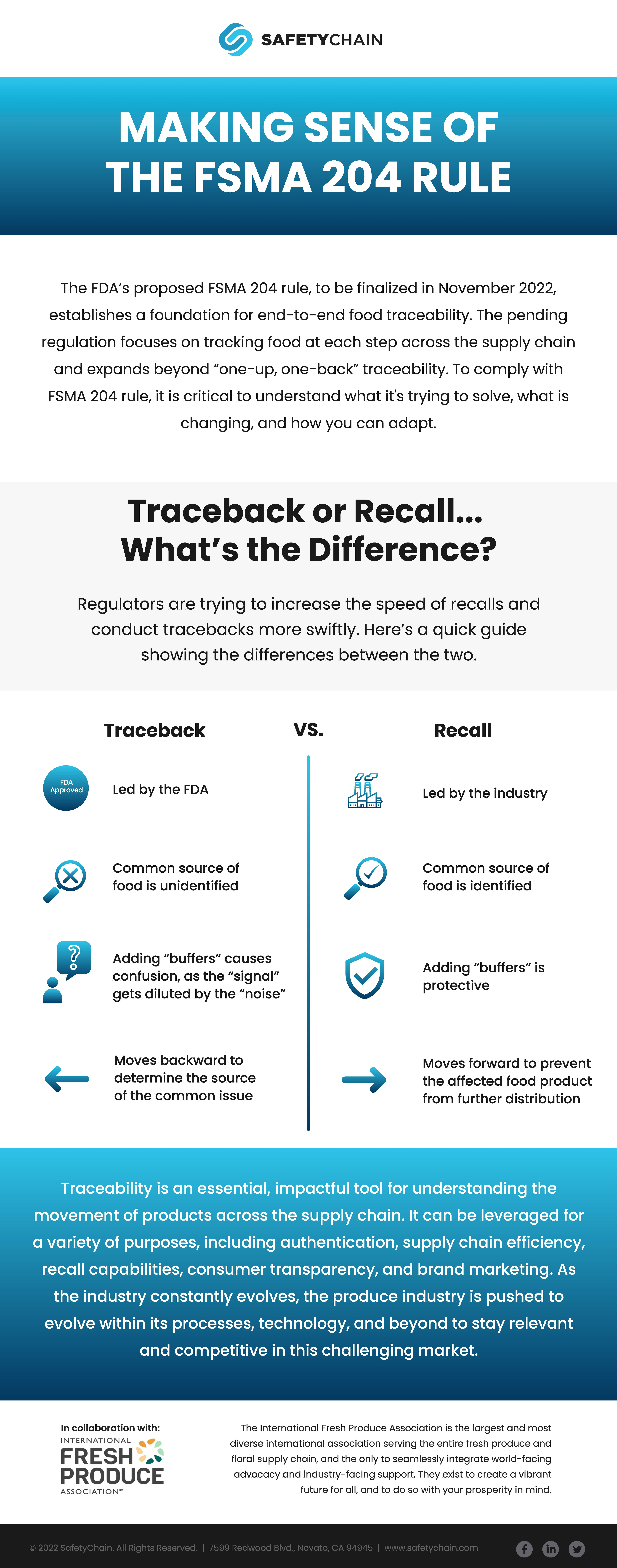
The approach to traceability in FSMA 204 replaces the method that many producers have used of tracing “one up and one back” within the supply chain. As global supply chains have increased in complexity, it has become much more challenging for government agencies and producers within the industry to trace issues along the supply chain effectively.
Any facility that holds, packs, manufactures, or processes one or more foods on the FTL must keep records of any Key Data Elements (KDEs) resulting from Critical Tracking Events (CTEs). While only those who deal with foods on the list are mandated, the FDA is encouraging others to adopt the same traceability processes.
Let’s take a look at the goals of traceability as the industry continues to evolve.
The goals of end-to-end traceability
At the same time that supply chains are becoming more complex, the need for a faster response is increasing. The goal of 204 is to help agencies and producers create pathways for a speedier response through tracebacks and recalls, leading to more time- and cost-efficient responses that lead to safer health outcomes.
From the publishing of Rule 204 in November 2022, organizations will have two years to come into compliance. However, the longer a company waits, the more challenging it will be to be fully compliant by 2025. In addition, all traceability solutions are not created equal, and selecting improper methods for your organization could end up costing more than it helps.
Digital transformation in recordkeeping
Organizations that rely on manual paperwork can still submit paperwork to the FDA this way but may find it overwhelming to track the sheer volume of data required by the new traceability rules. For example, a facility must keep Key Data Elements (KDEs) for each Critical Tracking Event. But if the facility handles multiple foods on the Food Traceability List, it will quickly generate an enormous volume of data. A facility must create KDEs for each of these CTEs:
- Growing
- Receiving, including First Receiver
- Creating
- Transformation
- Shipping
For many companies, this rule and the potential volume of data collection it requires has spurred them to either begin or continue the digitization of their processes. In addition, automation of KDE collection is paramount to more efficient traceability compliance.
Organizations will also have to keep traceability program records for regulators to ensure compliance. The ability to respond quickly is an important goal of FSMA 204, and facilities will have to respond to the FDA with traceability records within 24 hours of a request. Companies will also have to deliver a sortable electronic spreadsheet to the FDA within 24 hours when the FDA is investigating a public health threat or outbreak. Organizations can better prepare for multiple aspects of FSMA 204 compliance by transitioning to a food management software platform.
Don’t wait to prepare for FSMA 204
Organizations can leverage traceability as an impactful tool that helps them understand how products move along the supply chain. Governmental agencies, organizations within the industry, and consumers can benefit from the transparency and visibility that FSMA 204 hopes to deliver. As the global supply chain grows and changes, the industry must evolve its technology, processes, and approach to remain relevant and competitive. The finalization of 204 is coming quickly, and the food list and traceability aspects will likely continue to expand in the coming years. Proactively preparing your organization now can have a significant, positive impact over the long term.